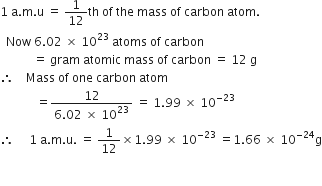
Atomic Mass Unit (abbreviations: amu, or u): also known as dalton (symbol: Da), is a standard unit of mass that used on an atomic or molecular scale (atomic mass).One unified atomic mass unit is about the mass of one nucleon (either a single proton or neutron) and equivalent to 1 g/mol. Kilogram (abbreviations: kg, or kgm): is a SI (metric) system unit of mass (IPK, also known as 'Le Grand K. Atomic mass unit (amu; dalton): A unit of mass equal to exactly 1/12 the mass of one 12 C atom, or 1.660538921 x 10 −27 kg. This is the approximate mass of one proton or one neutron. It is the basis of atomic weights. Convert the AMU value to grams by multiplying it by 1.67 x 10^-24. For example, 6 x 10^23 AMU times 1.67 x 10^-24 yields 1 gram. Find the molar weight (grams per mole) of the atom on the periodic table (see Resources). For example, oxygen's molar weight is approximately 16. One atomic mass unit (amu) is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Number of particles in one mole of any substance is equal to avogadro constant (N=6.023.10^23). So, mass of one nucleon (1 amu) = 1/N=1.6605.10^-24 grams. Neutral charge mass of 1 amu. Negative charge 0 amu. The number of protons is equal to. Most mass in an atom is in the.
››Convert dalton to atomic mass unit [1960]
Please enable Javascript to usethe unit converter.
Note you can turn off most ads here:
https://www.convertunits.com/contact/remove-some-ads.php
| Did you mean to convert | dalton | to | atomic mass unit [1960] atomic mass unit [1973] atomic mass unit [1986] atomic mass unit [1998] |
››More information from the unit converter
How many dalton in 1 amu?The answer is 0.99986408037577.
We assume you are converting between dalton and atomic mass unit [1960].
You can view more details on each measurement unit:
dalton oramu
The SI base unit for mass is the kilogram.
1 kilogram is equal to 6.0221366516752E+26 dalton, or 6.0229552894949E+26 amu.
Note that rounding errors may occur, so always check the results.
Use this page to learn how to convert between daltons and atomic mass units.
Type in your own numbers in the form to convert the units!
1 Amu Is Equal To The Mass Of One
››Quick conversion chart of dalton to amu
1 dalton to amu = 1.00014 amu
5 dalton to amu = 5.00068 amu
Parallels support for mac. 10 dalton to amu = 10.00136 amu
15 dalton to amu = 15.00204 amu
20 dalton to amu = 20.00272 amu
25 dalton to amu = 25.0034 amu
30 dalton to amu = 30.00408 amu
40 dalton to amu = 40.00544 amu
50 dalton to amu = 50.0068 amu
››Want other units?
You can do the reverse unit conversion fromamu to dalton, or enter any two units below:
››Common weight conversions
How To Find Amu Chemistry

dalton to denier
dalton to metric ton
dalton to uncia
dalton to scruple
dalton to electron
dalton to tahil
dalton to quarter (ton)
dalton to kilo
dalton to maund
dalton to ton
Hp drivers software for mac.
››Metric conversions and more
ConvertUnits.com provides an onlineconversion calculator for all types of measurement units.You can find metric conversion tables for SI units, as wellas English units, currency, and other data. Type in unitsymbols, abbreviations, or full names for units of length,area, mass, pressure, and other types. Examples include mm,inch, 100 kg, US fluid ounce, 6'3', 10 stone 4, cubic cm,metres squared, grams, moles, feet per second, and many more!

Click to see full answer.
In this regard, what is the energy equivalent of 1 amu?
Since 1 amu is equivalent to 931.5 MeV of energy, the BE can be calculated using Equation 8.6. Example: Calculate the mass defect and BE for U-235.
Subsequently, question is, is Amu equal to g mol? amu vs.The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units (amu) or in grams per mole (g/mol). Although mass can be expressed as both amu and g/mol, g/mol is the most useful system of units for laboratory chemistry.
how do you convert AMU to MeV?
1 Amu Is Equal To Mev
Multiplying the value of 1 amu (in grams) by value of c² ( square of the velocity of light in vaccum) we shall get the value of 1 amu in ergs (a unit of energy). We can convert ergs in terms of electron volt ( 1 electron volt abbreviated as eV = 1.6 × 10^-12 ergs). Doing this we get 1 amu = 931.5 ×10^6 eV = 931.5 MeV.
What is MeV c2?
Million Electron Volts /Charge square. Well, MeV/c^2 is a unit of mass, and MeV is a unit of energy. The rest energy of a particle can be computed in units of MeV by multiplying it's rest mass in units of MeV/c^2 by c^2.
